Abstract
Background: The main treatment goal for patients (pts) with newly diagnosed Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia in chronic phase (CML-CP) is disease control. Standard therapy is with any of the tyrosine kinase inhibitors (TKIs) approved for 1st-line (1L) use: the 1st-generation TKI imatinib (IMA) or one of the 2nd-generation (2G) TKIs bosutinib (BOS), dasatinib (DAS), and nilotinib (NILO). However, in 1L CML-CP, >50% of pts treated with IMA develop resistance to or intolerance of therapy, and 30% to 40% of pts treated with a 2G TKI need to switch therapy by 5 years. In addition, only 30% to 55% of pts achieve a deep molecular response (DMR); needed criterion for attempting treatment-free remission. While 2G TKIs yield a higher major molecular response (MMR) than IMA, they are associated with more adverse events (AEs), including arterio-occlusive events. Therefore, treatment options with improved efficacy and tolerability are needed for pts in 1L CML-CP.
Asciminib is the first BCR::ABL1 inhibitor that works by Specifically Targeting the ABL Myristoyl Pocket (STAMP) and has been recently approved by the FDA for Ph+ CML-CP patients treated with ≥2 prior TKIs. In the pivotal phase III ASCEMBL trial in pts treated with ≥2 prior TKIs, after over 2 years of follow-up, asciminib demonstrated sustained superior efficacy, safety and tolerability compared with bosutinib. Here we present a phase III trial evaluating asciminib vs an investigator-selected approved TKI in adult pts with newly diagnosed Ph+ CML-CP.
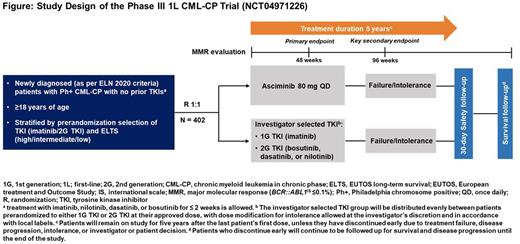
Methods: ASC4FIRST (NCT04971226) is a multicenter, open-label, randomized, phase III study of asciminib 80 mg once daily (QD) monotherapy compared with an approved, investigator-selected TKI (~50% IMA; ~50% either BOS, or DAS, or NILO) for adult pts with newly diagnosed Ph+ CML-CP (expected N=402).
Eligible pts must not have received prior TKI therapy for CML except for ≤2 weeks of either IMA, NILO, DAS, or BOS and must have Eastern Cooperative Oncology Group performance status of 0 or. Pts could have received prior hydroxyurea (HU) and/or anagrelide treatment. Pts will be randomized 1:1 to asciminib or investigator-selected TKI using 2 strata: European Treatment and Outcome Study Long-Term Survival (ELTS) score and the control arm TKI selected by the investigator before randomization (Figure). Pts randomized to the investigator selected TKI arm will receive their selected TKIs at approved doses: IMA 400 mg QD, BOS 400 mg QD, DAS 100 mg QD, or NILO 300 mg twice daily. Dose recommendations as per local label should be followed for participants with hepatic and renal impairment.
The primary objectives are to compare the efficacy of asciminib vs investigator-selected TKIs and to compare the efficacy of asciminib vs IMA in the stratum of investigator-selected IMA. The primary endpoint is MMR at week 48. The study will be positive if either of these objectives is met. The key secondary endpoint is MMR at week 96. Additional secondary endpoints include time to discontinuation of study treatment due to an AE (TTDAE); MMR, MR4, MR4.5, complete hematological response (CHR) and BCR::ABL1 ≤1% at and by all scheduled data collection time points; duration of and time to first MMR, MR4 and MR4.5; time to treatment failure; event-free survival, failure-free survival, progression-free survival, and overall survival.
Pts will remain on treatment for 5 years after the last pt.'s first treatment has been administered, unless they have discontinued early due to treatment failure, disease progression, intolerance, or investigator/pt. decision. All pts including those who discontinue early will continue to be followed up for survival and disease progression until the end of the study.
Current Status: As of 18th July 2022, 203 participants have been randomized and are receiving treatment. This Novartis-sponsored study is ongoing.
Disclosures
Hughes:Novartis: Consultancy, Research Funding; Enliven: Consultancy, Research Funding; BMS: Consultancy, Research Funding. Cortes:Takeda: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Sun Pharma: Consultancy, Research Funding; Biopath Holdings Inc: Consultancy, Current equity holder in private company; Abbvie: Consultancy, Research Funding; Forma Therapeutic: Consultancy; Gilead: Consultancy. Takahashi:Novartis: Consultancy, Honoraria, Research Funding; Otsuka Pharmaceutical: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria; Asahi-kasei: Research Funding. Larson:Servier: Consultancy; Novartis: Consultancy, Research Funding; MorphoSys: Consultancy; MedPace: Consultancy; CVS/caremark: Consultancy; BMS: Consultancy; Immunogen: Consultancy; Astellas: Consultancy, Research Funding; Takeda: Consultancy; Epizyme: Consultancy; Amgen: Consultancy; Daiichi Sankyo: Research Funding; Rafael Pharmaceuticals: Research Funding; Gilead: Research Funding; Celgene: Research Funding; cellectis: Research Funding. Issa:Novartis, Kura Oncology, Nuprobe: Consultancy; Celgene, Kura Oncology, Syndax, Merck, Cullinan and Novartis: Research Funding. Bombaci:Novartis: Consultancy. Ramscar:Novartis: Current Employment. Kapoor:Novartis: Current Employment, Current equity holder in private company. Ifrah:Novartis: Current Employment. Hochhaus:Bristol Myers Squibb: Research Funding; Novartis: Research Funding; Pfizer: Research Funding; Incyte: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal